Graphite lubrication now viable for rolling bearings
04.11.2022Oil is a well-known and widely used lubricant—less commonplace, however, is the solid lubricant graphite. Although graphite is used at low-contact pressure, the effects that occur here had previously only been understood to a limited extent. It was particularly unclear whether this lubricant could also be used at high-contact pressure, such as it occurs in rolling bearings. Researchers were now able to expand the current friction model to explain these effects. In a recently published article in the journal Nature Communications, they specify how their findings pave the way for the future use of graphite lubrication in high-pressure applications.
Whether in car motors or bicycle chains: Gearboxes, bearings, chains and the like are often lubricated with oil to minimize friction and wear. Yet oil is not always the lubricant of choice: Harsh conditions, high temperatures and occasionally even the food processing industry require solid lubricants. In cases such as these, graphite lubrication has become a well known alternative to oil. Just as it is employed in generators and airplanes, graphite can be used to help a stubborn key turn in its lock. All of these applications have one thing in common, however: They involve friction processes in which only low-contact pressure is present.
Detecting high pressures
But how does graphite lubrication behave at a high-contact pressure of several gigapascals, such as it occurs in rolling bearings? Researchers at the Fraunhofer Institute for Mechanics of Materials IWM and the Karlsruhe Institute of Technology (KIT) sought to answer this question. Furthermore, they examined the effect of air humidity in graphite lubrication as the lubrication becomes ineffective at insufficient humidity, and the two friction contacts are instead cold-welded together. So far, Savage’s physical adsorption model has provided a rough explanation for this phenomenon: According to this model, the uppermost graphite layer is saturated with water and thus passivated. The usually reactive graphite then becomes inert.
Via experiments…
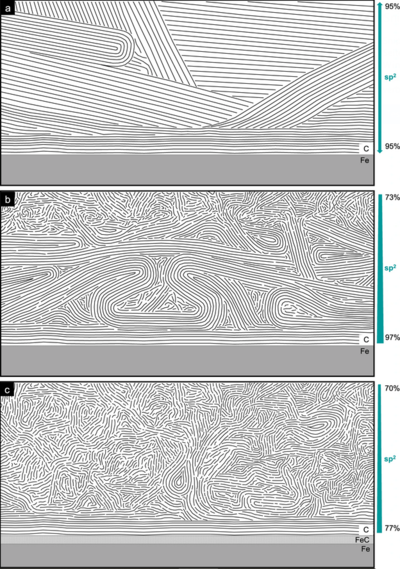
“While Savage’s adsorption model was generally accepted, it was tested at very low surface pressures,” says Carina Morstein, a scientist in the group “Applied Nanotribology” at the Institute of Applied Materials (IAM) at KIT. “We therefore conducted experiments at both very high and very low contact pressures.” In a series of tests, Morstein exposed the graphite layers on the friction bodies to different pressures and produced cross-sections, which she then examined with a transmission microscope. “Before the layers are exposed to the pressure of friction, it is possible to make out long lamellae in the graphite layer. After undergoing pressure and sliding, however, these lamellae disappear, leaving behind graphite swirls that somewhat resemble a Van Gogh painting,” Morstein explains. “The shear in frictional contact therefore causes a structural change from the polycrystalline system to turbostratic carbon, which was not taken into account in previous models,” says Prof. Dr. Martin Dienwiebel of IAM.
…and quantum-chemical simulations
Researchers at Fraunhofer IWM confirmed this observation with quantum-chemical simulations: Here, too, the graphite crystals do not remain stable under pressure but form curved loops instead. However, as predicted by the Savage model, the uppermost layers become saturated with water. “Savage’s model is a good starting point, but it does not take consider the reordering of graphite. Instead of graphite sliding on graphite, turbostratic carbon slides on turbostratic carbon,” says Prof. Dr. Michael Moseler, who manages the group “Multiscale Modeling and Tribosimulation” at Fraunhofer IWM. This turbostratic layer forms even at low loads: the higher the pressure, the greater the graphite’s transformation, with the swirled layers growing correspondingly thicker. “In expanding the current model of graphite lubrication, we have laid a better foundation for understanding it. This opens up the possibility for numerous new applications,” concludes Morstein. The team’s findings were recently published in the journal Nature Communications.
The project’s outcome raises an important question: Can the lubrication work despite the transformation of the lubricating graphite layer on the friction partners? “Absolutely,” says Moseler. “The lubrication is effective at both low and high pressures. We were able to demonstrate for the first time that graphite lubrication, for example, can also be used in axial bearings.” The researchers plan to investigate exactly what this might look like in a follow-up project and ultimately hope to develop a graphite-lubricated axial rolling bearing.
Publication
Morstein, C.E.; Klemenz, A.; Dienwiebel, M.; Moseler, M., Humidity-dependent lubrication of highly loaded contacts by graphite and a structural transition to turbostratic carbon, Nature Communications 13 (2022) Art. 5958, 16 pp. Link (Nature Communications)Link (Fraunhofer-Publica)
Source: Press Release Fraunhofer Institute for Mechanics of Materials IWM